Textbook Question
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. c. 5.5% NaNO3 by mass (in water)
2334
views

 Verified step by step guidance
Verified step by step guidance

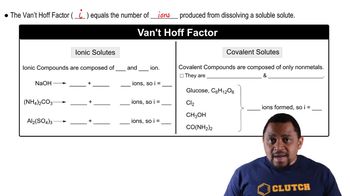

Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. c. 5.5% NaNO3 by mass (in water)
What mass of salt (NaCl) should you add to 1.00 L of water in an ice cream maker to make a solution that freezes at -10.0 °C? Assume complete dissociation of the NaCl and density of 1.00 g/mL for water.
A 1.2 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 101.4 °C. Calculate the van't Hoff factor (i) for MX2 at this concentration.
A 0.100 M ionic solution has an osmotic pressure of 8.3 atm at 25 °C. Calculate the van't Hoff factor (i) for this solution.