
 Sanders 3rd Edition
Sanders 3rd Edition Ch. 20 - Population Genetics and Evolution at the Population, Species, and Molecular Levels
Ch. 20 - Population Genetics and Evolution at the Population, Species, and Molecular Levels Problem 17
Problem 17Genetic Analysis 20.1 predicts the number of individuals expected to have the blood group genotypes MM, MN, and NN. Perform a chi-square analysis using the number of people observed and expected in each blood-type category, and state whether the sample is in H-W equilibrium.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
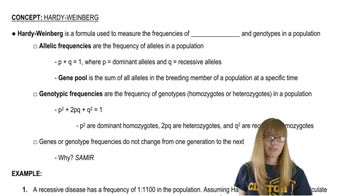
Key Concepts
Blood Group Genotypes

Chi-Square Analysis

Hardy-Weinberg Equilibrium
Certain animal species, such as the black-footed ferret, are nearly extinct and currently exist only in captive populations. Other species, such as the panda, are also threatened but exist in the wild thanks to intensive captive breeding programs. What strategies would you suggest in the case of black-footed ferrets and in the case of pandas to monitor and minimize inbreeding depression?
In a population of rabbits, f(C₁) = 0.70 and f(C₂) = 0.30. The alleles exhibit an incomplete dominance relationship in which C₁C₁ produces black rabbits, C₁C₂ produces tan-colored rabbits, and C₂C₂ produces rabbits with white fur. If the assumptions of the Hardy–Weinberg principle apply to the rabbit population, what are the expected frequencies of black, tan, and white rabbits?
Sickle cell disease (SCD) is found in numerous populations whose ancestral homes are in the malaria belt of Africa and Asia. SCD is an autosomal recessive disorder that results from homozygosity for a mutant β-globin gene allele. Data on one affected population indicates that approximately 8 in 100 newborn infants have SCD.
What are the frequencies of the wild-type (βᴬ) and mutant (βˢ) alleles in this population?
Sickle cell disease (SCD) is found in numerous populations whose ancestral homes are in the malaria belt of Africa and Asia. SCD is an autosomal recessive disorder that results from homozygosity for a mutant β-globin gene allele. Data on one affected population indicates that approximately 8 in 100 newborn infants have SCD.
What is the frequency of carriers of SCD in the population?