Textbook Question
Draw structures corresponding to the following names:
a. 2,4-Dimethyl-2-heptanol
840
views

 Verified step by step guidance
Verified step by step guidance

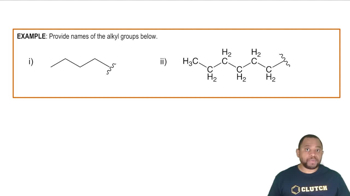

Draw structures corresponding to the following names:
a. 2,4-Dimethyl-2-heptanol
Draw structures corresponding to the following names:
b. 2,2-Diethylcyclohexanol
Draw structures corresponding to the following names:
f. 3,3-Dimethyl-1,6-heptanediol
Draw structures corresponding to the following names:
e. 2,4-Dimethoxy-3-methylpentane
Draw structures corresponding to the following names:
f. 3-Methoxy-4-methyl-1-pentene
Identify each alcohol named in Problem 14.32 as primary, secondary, or tertiary.
a.
b.
c.
d.
e.
f.