Textbook Question
How many NADH and how many FADH2 molecules are formed in the citric acid cycle?
1068
views

 Verified step by step guidance
Verified step by step guidance
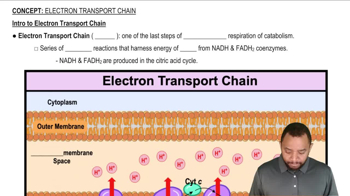
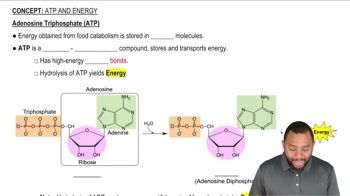
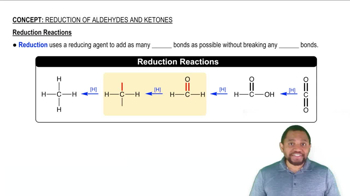
How many NADH and how many FADH2 molecules are formed in the citric acid cycle?
Which reactions of the citric acid cycle transfer energy as FADH2?
Which reactions of the citric acid cycle transfer energy as NADH?
What two coenzymes are involved with initial events of the electron-transport chain?
What are the ultimate products of the electron-transport chain?
What do the following abbreviations stand for?
b. CoQ