Textbook Question
Identify the following carbohydrates as the ⍺ or β anomer:
(a)
582
views

 Verified step by step guidance
Verified step by step guidance

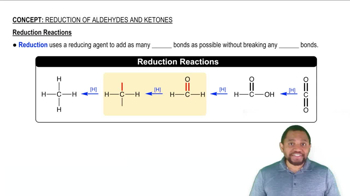

Identify the following carbohydrates as the ⍺ or β anomer:
(a)
Identify the following carbohydrates as the ⍺ or β anomer:
(b)
Draw the Fischer projection of the product of the oxidation of D-galactose at C1.
Will the following carbohydrates produce a positive Benedict's test?
a. D-glucose
Will the following carbohydrates produce a positive Benedict’s test?
b. lactose
Draw the product of the following 1→4 condensation and name the glycosidic bond: