Textbook Question
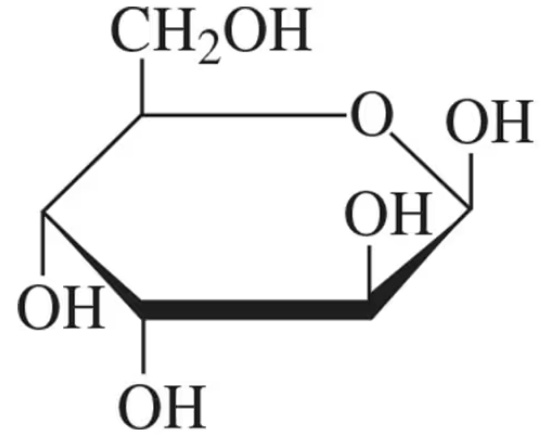
How are the following pairs of carbohydrates, shown in a Fischer projection, related to each other? Are they structural isomers, enantiomers, diastereomers, or epimers?
(a)
573
views

 Verified step by step guidance
Verified step by step guidance



How are the following pairs of carbohydrates, shown in a Fischer projection, related to each other? Are they structural isomers, enantiomers, diastereomers, or epimers?
(a)
Draw the Fischer projection of the C3 epimer of D-glucose. Compare your structure with those in Table 6.1 and give the name of this compound.
Identify the following carbohydrates as the ⍺ or β anomer:
(a)
Draw the Fischer projection of the product of the oxidation of D-galactose at C1.
Draw the Fischer projection of the product of reduction reaction of D-galactose at C1.
Will the following carbohydrates produce a positive Benedict's test?
a. D-glucose