Draw structures corresponding to the following names:
f. 3-Methoxy-4-methyl-1-pentene

 Verified step by step guidance
Verified step by step guidance
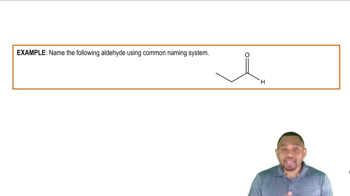


Draw structures corresponding to the following names:
f. 3-Methoxy-4-methyl-1-pentene
Identify each alcohol named in Problem 14.32 as primary, secondary, or tertiary.
a.
b.
c.
d.
e.
f.
Arrange the following 6-carbon compounds in order of their expected boiling points, and explain your ranking:
a. Hexane
b. 1-Hexanol
c. Dipropyl ether (CH3CH2CH2—O—CH2CH2CH3)
What type of product is formed on reaction of an alcohol with Na metal?
Assume that you have samples of the following two compounds, both with formula C7H8O. Both compounds dissolve in ether, but only one of the two dissolves in aqueous NaOH. How could you use this information to distinguish between them?
Which of the following alcohols can undergo oxidation? Draw the line structure of the product expected for those that can. Assume an excess of oxidizing agent is present.
a.
b.
c.