Textbook Question
Use the kinetic molecular theory of gases to explain each of the following:
c. Gases have low densities.
812
views

 Verified step by step guidance
Verified step by step guidance

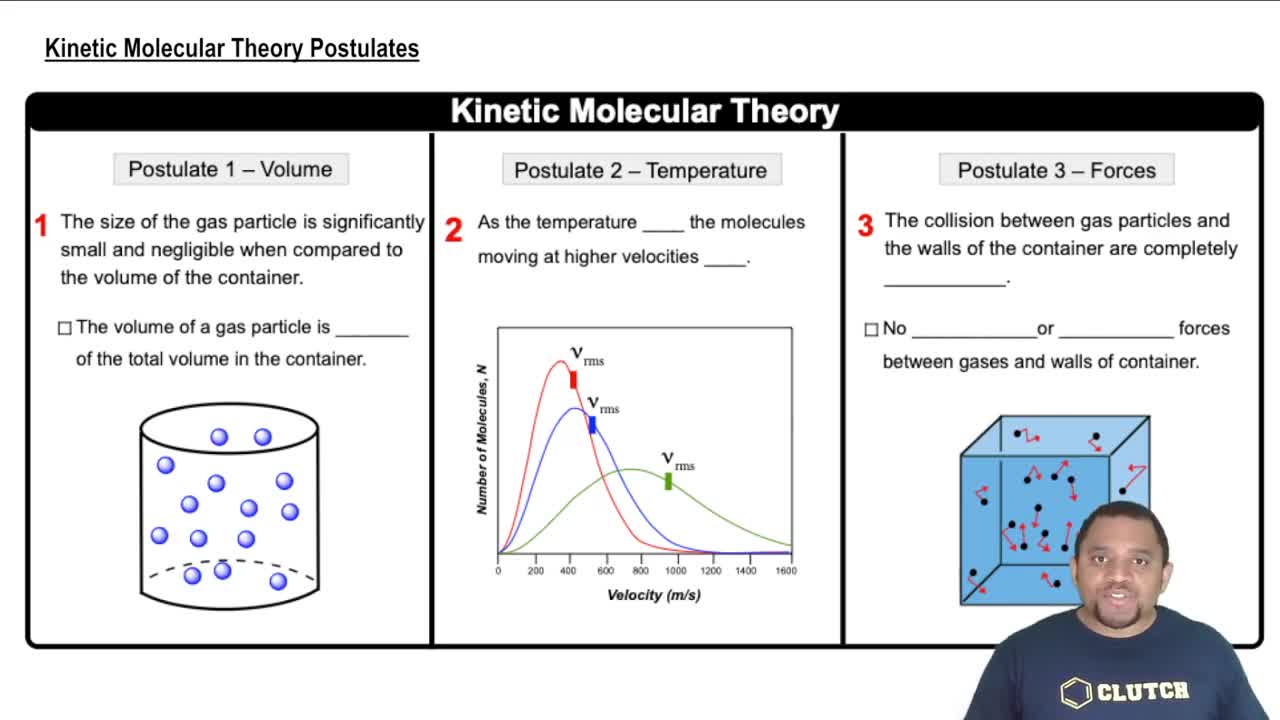
Use the kinetic molecular theory of gases to explain each of the following:
c. Gases have low densities.
Use the kinetic molecular theory of gases to explain each of the following:
c. You can smell the odor of cooking onions from far away.
A tank contains oxygen (O2) at a pressure of 2.00 atm. What is the pressure in the tank in terms of the following units?
b. lb/in.2
A tank contains oxygen (O2) at a pressure of 2.00 atm. What is the pressure in the tank in terms of the following units?
d. kPa