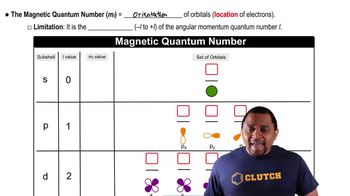
Azimuthal Quantum Number (l)
The azimuthal quantum number, denoted as 'l', determines the shape of an electron's orbital. It can take integer values from 0 to n-1, where n is the principal quantum number. For example, if l = 0, the orbital is spherical (s), and if l = 2, the orbital has a cloverleaf shape (d), which corresponds to four lobes.

 Verified step by step guidance
Verified step by step guidance