15. Chemical Kinetics
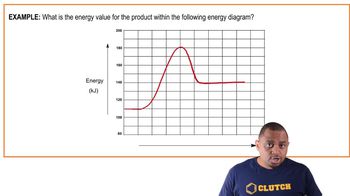
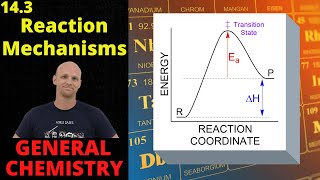
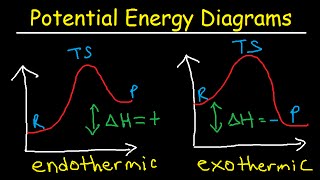
Energy Diagrams

Get help from an AI Tutor
Ask a question to get started.
Problem 92b
Textbook Question
Textbook QuestionConsider three reactions with different values of Ea and ΔE: Reaction 1. Ea = 20 kJ>mol; ΔE = -60 kJ>mol Reaction 2. Ea = 10 kJ>mol; ΔE = -20 kJ>mol Reaction 3. Ea = 40 kJ>mol; ΔE = +15 kJ>mol (b) Assuming that all three reactions are carried out at the same temperature and that all three have the same frequency factor A, which reaction is the fastest and which is the slowest?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
330
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos