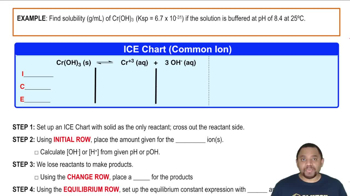
Textbook Question
Consider a beaker containing a saturated solution of CaF2 in equilibrium with undissolved CaF21s2. Solid CaCl2 is then added to the solution. (a) Will the amount of solid CaF2 at the bottom of the beaker increase, decrease, or remain the same?
1186
views