Textbook Question
An element crystallizes in a face-centered cubic lattice. The edge of the unit cell is 4.078 Å, and the density of the crystal is 19.30 g>cm3. Calculate the atomic weight of the element and identify the element.
1518
views
1
rank

 Verified step by step guidance
Verified step by step guidance

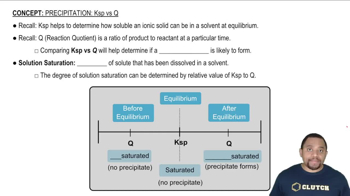
An element crystallizes in a face-centered cubic lattice. The edge of the unit cell is 4.078 Å, and the density of the crystal is 19.30 g>cm3. Calculate the atomic weight of the element and identify the element.
Determine if each statement is true or false: (b) Substitutional alloys have 'solute' atoms that replace 'solvent' atoms in a lattice, but interstitial alloys have 'solute' atoms that are in between the 'solvent' atoms in a lattice.
For each of the following alloy compositions, indicate whether you would expect it to be a substitutional alloy, an interstitial alloy, or an intermetallic compound: (a) Fe0.97Si0.03 (b) Fe0.60Ni0.40 (c) SmCo5.