Textbook Question
(a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days?
493
views

 Verified step by step guidance
Verified step by step guidance

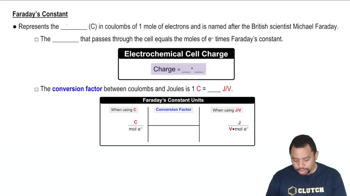

(a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days?
(b) What amperage is required to plate out 0.250 mol Cr from a Cr3+ solution in a period of 8.00 h?
A disproportionation reaction is an oxidation–reduction reaction in which the same substance is oxidized and reduced. Complete and balance the following disproportionation reactions:
(b) MnO42-(aq) → MnO4-(aq) + MnO2(s) (acidic solution)