Textbook Question
Indicate the number of protons and neutrons in the following nuclei: (c) neptunium-237.
572
views

 Verified step by step guidance
Verified step by step guidance
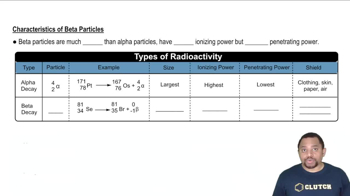


Indicate the number of protons and neutrons in the following nuclei: (c) neptunium-237.
Give the symbol for (b) an alpha particle.
Give the symbol for (c) gamma radiation.
Give the symbol for (c) a positron.
Write balanced nuclear equations for the following processes: (a) rubidium-90 undergoes beta emission.
Write balanced nuclear equations for the following processes: (b) selenium-72 undergoes electron capture.