Pakistan's K2 is the world's second-tallest mountain, with an altitude of 28,251 ft. Its base camp, where climbers stop to acclimate, is located about 16,400 ft above sea level. (a) Approximate atmospheric pressure P at different altitudes is given by the equation P = e-h/7000, where P is in atmospheres and h is the altitude in meters. What is the approximate atmospheric pressure in mm Hg at K2 base camp? (b) What is the atmospheric pressure in mm Hg at the summit of K2?
Ch.10 - Gases: Their Properties & Behavior

Chapter 10, Problem 134
A driver with a nearly empty fuel tank may say she is 'running on fumes.' If a 15.0-gallon automobile gas tank had only gasoline vapor remaining in it, what is the farthest the vehicle could travel if it gets 20.0 miles per gallon on liquid gasoline? Assume the average molar mass of molecules in gasoline is 105 g/mol, the density of liquid gasoline is 0.75 g/mL, the pressure is 743 mm Hg, and the temperature is 25 °C.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Convert the pressure from mm Hg to atm. Use the conversion factor 1 atm = 760 mm Hg.
Step 2: Convert the temperature from Celsius to Kelvin. The formula to convert Celsius to Kelvin is K = °C + 273.15.
Step 3: Use the ideal gas law, PV = nRT, to calculate the number of moles of gasoline vapor in the tank. P is the pressure in atm, V is the volume of the tank in liters, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/K·mol), and T is the temperature in Kelvin.
Step 4: Convert the number of moles of gasoline vapor to grams using the molar mass of gasoline (105 g/mol).
Step 5: Convert the mass of gasoline vapor to volume of liquid gasoline using the density of gasoline (0.75 g/mL). Then, convert this volume to gallons using the conversion factor 1 gallon = 3785.41 mL. Finally, multiply this volume by the fuel efficiency of the car (20.0 miles/gallon) to find the maximum distance the car could travel.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
7mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of a gas through the equation PV = nRT. This law is essential for understanding how gas behaves under different conditions. In this scenario, it helps determine the volume of gasoline vapor that can be produced from the remaining gasoline in the tank, which is crucial for calculating the distance the vehicle can travel.
Recommended video:
Guided course

Ideal Gas Law Formula
Density and Volume Conversion
Density is defined as mass per unit volume and is crucial for converting between mass and volume. In this question, the density of liquid gasoline (0.75 g/mL) allows us to convert the mass of gasoline vapor into a volume that can be used in calculations. Understanding how to manipulate these units is key to finding the total volume of gasoline vapor available for combustion.
Recommended video:
Guided course
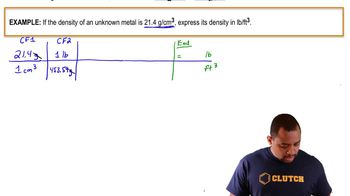
Density Conversion Example
Molar Mass and Stoichiometry
Molar mass is the mass of one mole of a substance, which is vital for converting between grams and moles. In this case, the average molar mass of gasoline (105 g/mol) is used to determine how many moles of gasoline vapor are present. This information is necessary for calculating the total energy available for combustion and, ultimately, the distance the vehicle can travel based on its fuel efficiency.
Recommended video:
Guided course

Molar Mass Concept
Related Practice
Textbook Question
832
views
Textbook Question
Pakistan's K2 is the world's second-tallest mountain, with an altitude of 28,251 ft. Its base camp, where climbers stop to acclimate, is located about 16,400 ft above sea level. (c) Assuming the mole fraction of oxygen in air is 0.2095, what is the partial pressure of oxygen in mm Hg at the summit of K2?
520
views
Textbook Question
Assume that you take a flask, evacuate it to remove all the air, and find its mass to be 478.1 g. You then fill the flask with argon to a pressure of 2.15 atm and reweigh it. What would the balance read in grams if the flask has a volume of 7.35 L and the temperature is 20.0 °C?
679
views
