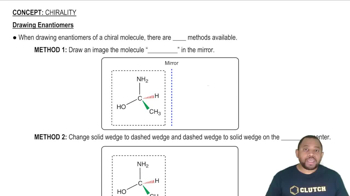
Textbook Question
Which of the following complexes can exist as enantiomers? Draw their structures.
(a) [Cr(en)3]3+
(b) cis-[Co(NH3)Cl]2+
(c) trans-[Co(en)2(NH3)Cl]2+
(d) [Pt(NH3)3Cl3]+
112
views

 Verified step by step guidance
Verified step by step guidance

Which of the following complexes can exist as enantiomers? Draw their structures.
(a) [Cr(en)3]3+
(b) cis-[Co(NH3)Cl]2+
(c) trans-[Co(en)2(NH3)Cl]2+
(d) [Pt(NH3)3Cl3]+
What is the crystal field energy level diagram for the complex [Fe(NH3)6]3+?
(a)
(b)
(c)
(d)
Draw all possible diastereoisomers of [Cr(C2O4)2(H2O)2]-. Which can exist as a pair of enantiomers?
What is a racemic mixture? Does it affect plane-polarized light? Explain.
Draw the structure of all isomers of the octahedral complex [NbX2Cl4]- (X- = NCS-), and identify those that are linkage isomers.
The glycinate anion, gly-= NH2CH2CO2 -, bonds to metal ions through the N atom and one of the O atoms. Using to represent gly-, sketch the structures of the four stereoisomers of Co(gly)3.