Textbook Question
Dimethylhydrazine, a colorless liquid used as a rocket fuel, is 40.0% C, 13.3% H, and 46.7% N. What is the empirical formula? (LO 3.11) (a) CH4N9 (b) CH2N (c) C2H4N (d) C2H5N2
1050
views

 Verified step by step guidance
Verified step by step guidance


Dimethylhydrazine, a colorless liquid used as a rocket fuel, is 40.0% C, 13.3% H, and 46.7% N. What is the empirical formula? (LO 3.11) (a) CH4N9 (b) CH2N (c) C2H4N (d) C2H5N2
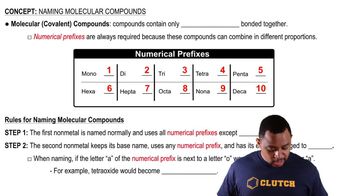
Combustion analysis is performed on 0.50 g of a hydrocar-bon and 1.55 g of CO2, and 0.697 g of H2O are produced. The mass spectrum for the hydrocarbon is provided below. What is the molecular formula? (LO 3.12 and 3.13)
(a) C5H11 (b) C8H18 (c) C11H10 (d) C10H22