Textbook Question
How many moles of solute are present in each of the following solutions? (a) 35.0 mL of 1.200 M HNO3
883
views

 Verified step by step guidance
Verified step by step guidance

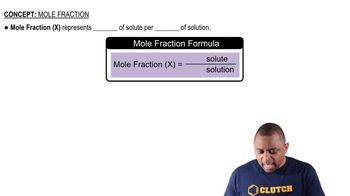

How many moles of solute are present in each of the following solutions? (a) 35.0 mL of 1.200 M HNO3
How many moles of solute are present in each of the following solutions? (b) 175 mL of 0.67 M glucose (C6H12O6)
How many grams of solute would you use to prepare each of the following solutions? (a) 250.0 mL of 0.600 M ethyl alcohol (C2H6O)
How many milliliters of a 0.45 M BaCl2 solution contain 15.0 g of BaCl2?