Textbook Question
When a copper salt such as Cu(NO3)2 is burned in a flame, a blue-green color is emitted. Which figure represents the emission spectrum for the element copper? (LO 5.6)? (a)
(b)
(c)
(d)
1133
views
1
rank

 Verified step by step guidance
Verified step by step guidance


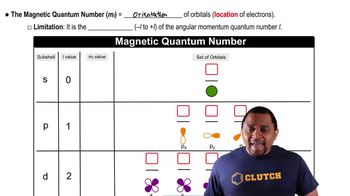
When a copper salt such as Cu(NO3)2 is burned in a flame, a blue-green color is emitted. Which figure represents the emission spectrum for the element copper? (LO 5.6)? (a)
(b)
(c)
(d)
Two electromagnetic waves are represented below. (
(a) Which wave has the greater intensity?
Two electromagnetic waves are represented below.
(c) Which wave represents yellow light, and which represents infrared radiation?