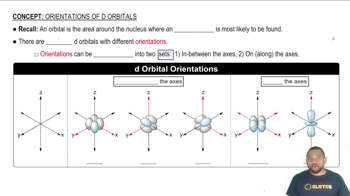
Transition Metals and d-Orbitals
Transition metals, such as titanium, have partially filled d-orbitals, which play a significant role in their chemical properties and electron configurations. The electron configuration of transition metals can be complex due to the involvement of d-orbitals, especially when forming ions. For Ti2+, the removal of electrons typically occurs from the 4s orbital before the 3d orbital, which is essential for accurately determining its electron configuration.

 McMurry 8th Edition
McMurry 8th Edition Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory
Ch.6 - Ionic Compounds: Periodic Trends and Bonding Theory Problem 42
Problem 42 Verified step by step guidance
Verified step by step guidance