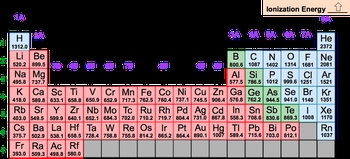
Ionization energy refers to the energy required to remove an electron from a gaseous atom or ion, measured in kilojoules. For example, when considering nitrogen in its gaseous state, the removal of an electron transforms it into a positively charged ion. This process can be represented by the equation:
N(g) + Energy → N+(g) + e-
In this reaction, energy is absorbed, indicating that ionization energy is always a positive value. This characteristic aligns with the definition of an endothermic reaction, where energy is taken in to break a bond. In this case, the bond being broken is the connection between the electron and the nitrogen atom.
Furthermore, it is important to understand that ionization energy is directly related to the potential energy of the electron being removed. As we delve deeper into the concepts of potential energy and ionization energy, we will explore how these ideas interconnect. For now, it is essential to recognize that the process of removing an electron results in the formation of a more positively charged species, highlighting the significance of ionization energy in chemical reactions.