Textbook Question
The reaction of A with B to give D proceeds in two steps: (1) A + B → C ΔH° = -20 kJ (2) C + B → D ΔH° = +50 kJ (3) A + 2B → D ΔH° = ? (b) What is the value of ΔH° for the overall reaction A + 2 B → D ΔH° = ?
363
views

 Verified step by step guidance
Verified step by step guidance


The reaction of A with B to give D proceeds in two steps: (1) A + B → C ΔH° = -20 kJ (2) C + B → D ΔH° = +50 kJ (3) A + 2B → D ΔH° = ? (b) What is the value of ΔH° for the overall reaction A + 2 B → D ΔH° = ?
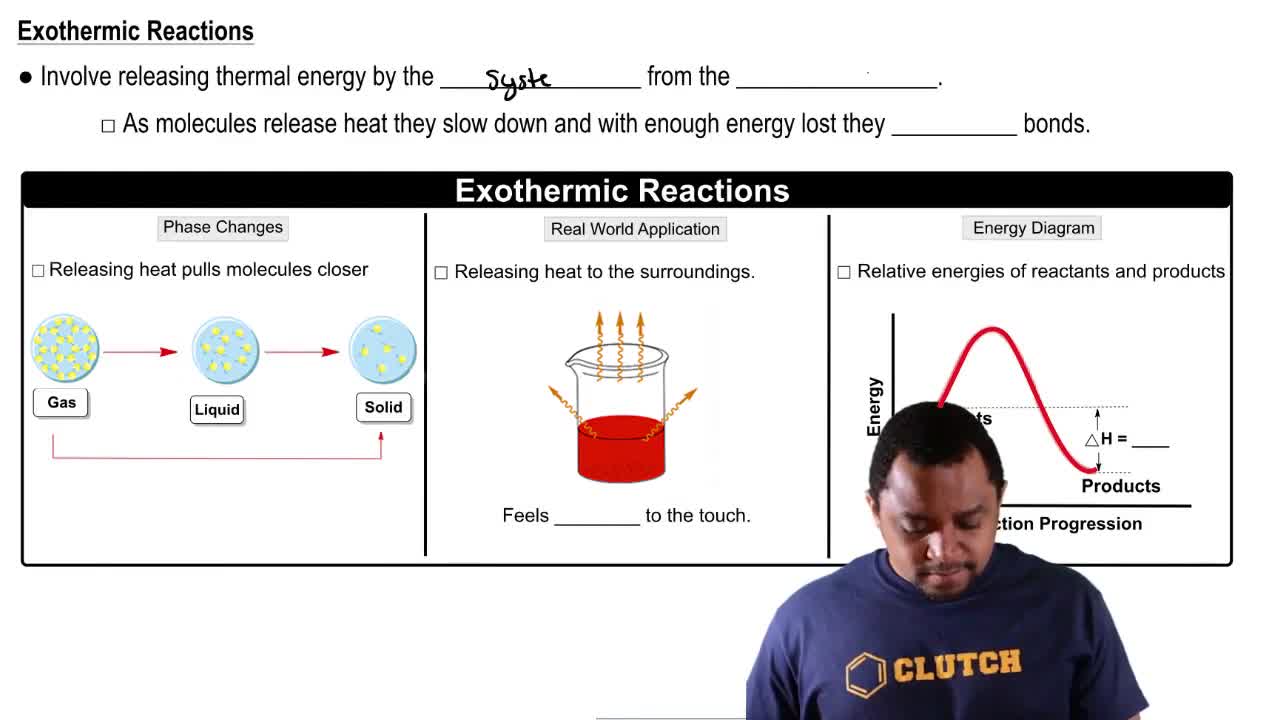
The following reaction is exothermic:
(a) Write a balanced equation for the reaction (red spheres represent A atoms and ivory spheres represent B atoms)
The following reaction of A3 molecules is spontaneous. (a) Write a balanced equation for the reaction.