Textbook Question
Name the straight-chain alkanes or cycloalkanes whose structure or formula is shown:
(b)
544
views

 Verified step by step guidance
Verified step by step guidance
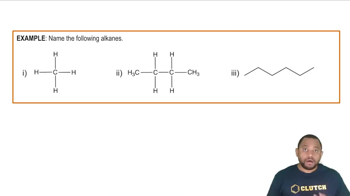
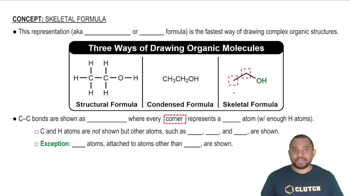

Name the straight-chain alkanes or cycloalkanes whose structure or formula is shown:
(b)
Write the condensed structure for the straight-chain alkanes shown:
(b) methane
Write the condensed structure for the straight-chain alkanes shown:
(c) hexane
Identify the family of hydrocarbon present in the following:
(a) H3CC≡CH
Identify the family of hydrocarbon present in the following:
(c)
Identify the family of hydrocarbon present in the following:
(a) CH3CH2CH=CH2