Textbook Question
Is each of the following statements true or false?
c. Neutrons repel each other.
1412
views

 Verified step by step guidance
Verified step by step guidance


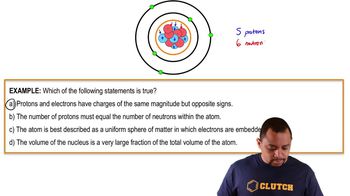
Is each of the following statements true or false?
c. Neutrons repel each other.
On a dry day, your hair flies apart when you brush it. How would you explain this?
Sometimes clothes cling together when removed from a dryer. What kinds of charges are on the clothes?
Would you use the atomic number, mass number, or both to determine each of the following?
d. number of electrons in a neutral atom
How many protons and electrons are there in a neutral atom of each of the following elements?
a. argon
Write the atomic symbol for the isotope with each of the following characteristics:
c. 25 electrons and 28 neutrons