Textbook Question
Why is CCl4 a nonpolar molecule, but is PCl3 a polar molecule?
1001
views

 Verified step by step guidance
Verified step by step guidance

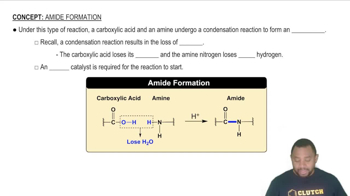
Why is CCl4 a nonpolar molecule, but is PCl3 a polar molecule?
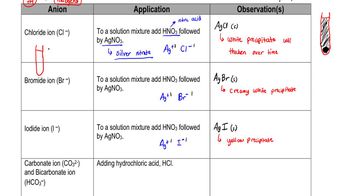
Identify the major type of intermolecular forces between the particles of each of the following:
b. MgF2
How does the octet rule explain the formation of a magnesium ion?
Why are Group 1A (1) and Group 2A (2) elements found in many compounds, but not Group 8A (18) elements?
Consider the following Lewis symbols for elements X and Y:
b. Will a compound of X and Y be ionic or molecular?
Consider the following Lewis symbols for elements X and Y:
c. What ions would be formed by X and Y?