Textbook Question
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
c. Na and Cl
2085
views

 Verified step by step guidance
Verified step by step guidance

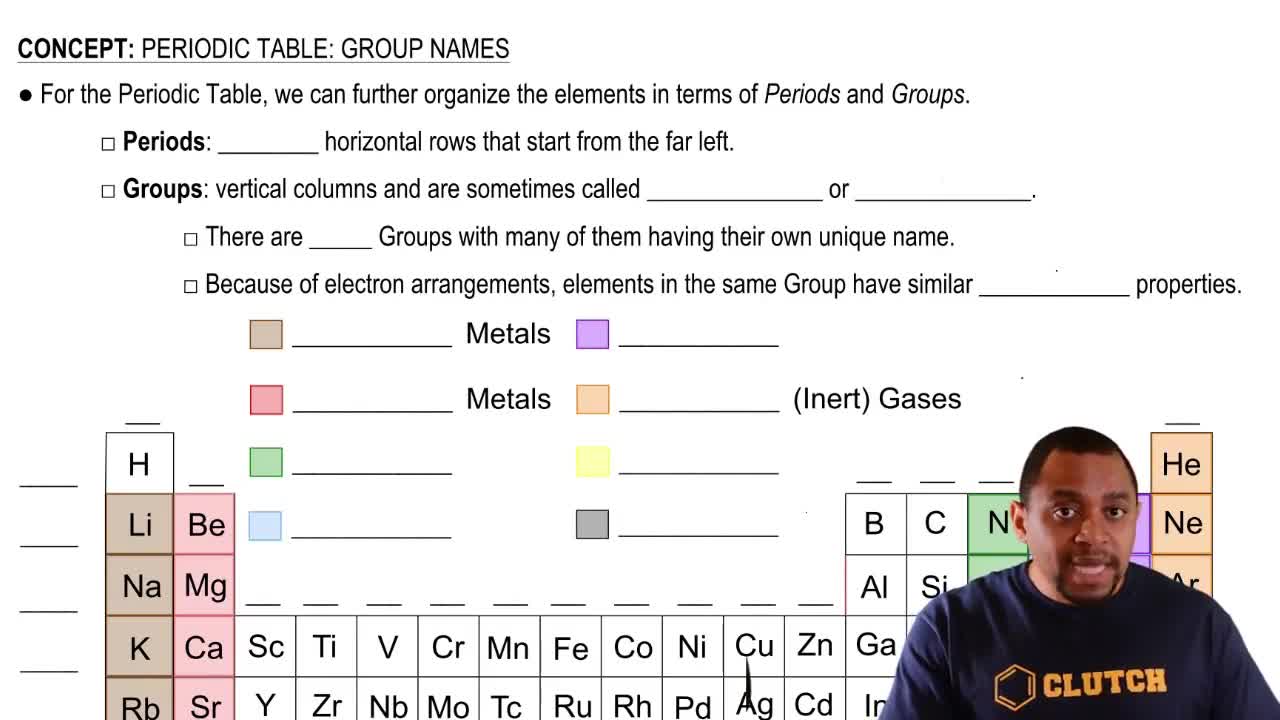
Calculate the electronegativity difference and classify each of the following bonds as nonpolar covalent, polar covalent, or ionic:
c. Na and Cl
Predict the shape and polarity of each of the following molecules, which have polar covalent bonds:
a. A central atom with three identical bonded atoms and one lone pair.
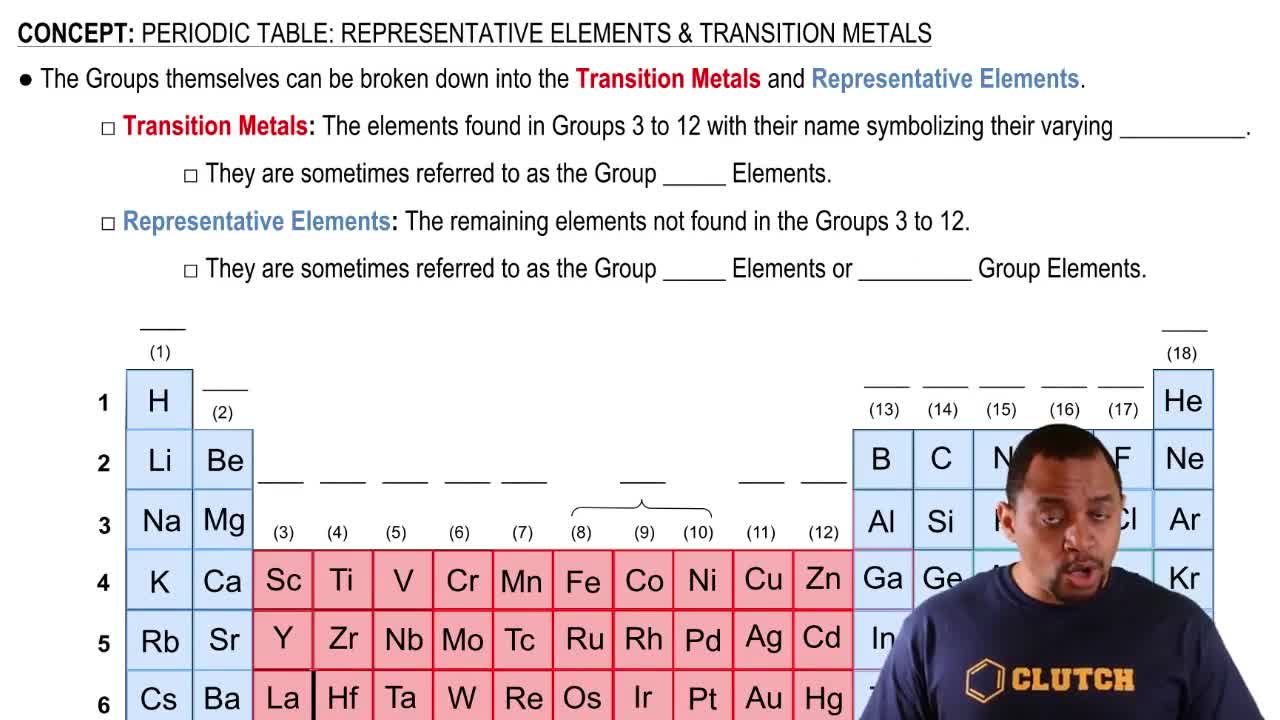
Identify the group number in the periodic table of X, a representative element, in each of the following ionic compounds:
a. XCl3
Identify the group number in the periodic table of X, a representative element, in each of the following ionic compounds:
b. X2SO3
Classify each of the following as ionic or molecular, and name each:
a. Li2HPO4
Classify each of the following as ionic or molecular, and name each:
b. Cl2O7