If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
b. write a balanced equation for the reaction.

 Verified step by step guidance
Verified step by step guidance



If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
b. write a balanced equation for the reaction.
If red spheres represent oxygen atoms, blue spheres represent nitrogen atoms, and all the molecules are gases,
c. indicate the type of reaction as combination, decomposition, single replacement, double replacement, or combustion.
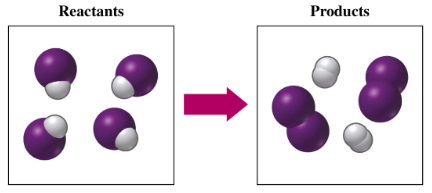
If purple spheres represent iodine atoms, white spheres represent hydrogen atoms, and all the molecules are gases,
a. write the formula for each of the reactants and products.
If purple spheres represent iodine atoms, white spheres represent hydrogen atoms, and all the molecules are gases,
c. indicate the type of reaction as combination, decomposition, single replacement, double replacement, or combustion.
Identify the type of reaction for each of the following as combination, decomposition, single replacement, double replacement, or combustion:
d. Zinc replaces copper in Cu(NO3)2.
Propane gas, C3H8, undergoes combustion with oxygen gas to produce carbon dioxide and water gases. Propane has a density of 2.02 g/L at room temperature.
a. Write the balanced chemical equation.