Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:
a. acetic acid, HC2H3O2, a weak electrolyte

 Verified step by step guidance
Verified step by step guidance
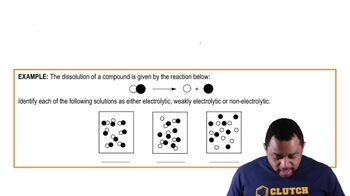
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:
a. acetic acid, HC2H3O2, a weak electrolyte
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:
b. NaBr, a strong electrolyte
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions: c. fructose, C6H12O6, a nonelectrolyte
Classify the solute represented in each of the following equations as a strong, weak, or nonelectrolyte:
b. NH3(g) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
Calculate the number of equivalents in each of the following:
d. 3 moles of CO32–
Calculate the number of equivalents in each of the following:
d. 2 moles of Fe3+