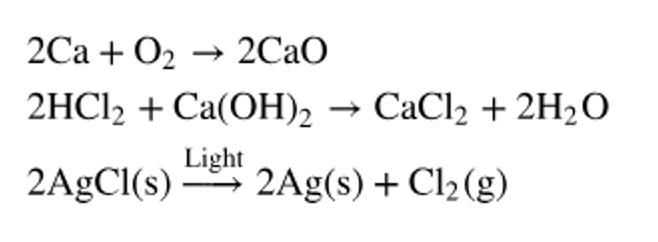Back
BackProblem 1
Using the periodic table, answer the following questions:
a. What is the atomic number for lithium (Li)?
b. What is the atomic mass of oxygen (O)?
c. What is the chemical symbol for potassium?
d. How many protons does nitrogen have?
e. How many neutrons does lead have?
Problem 2
What ions result when hydrogen donates one electron to fluorine?
Problem 3
Select all of the compounds from the following list. If it is not a compound, then state what it is.
a. H2O
b. HCO3-
c. O2
d. H2
e. Li2+
f. C6H12O6
g. H+
Problem 4
Determine the following:
a. The molarity of a solution with 0.5 moles of glucose per liter of water.
b. The concentration (in weight/volume percent) of a solution that contains 20 grams of sodium chloride per liter of water.
c. The concentration (in mg/dL) of a solution with 1 gram of lactic acid per 100 mL of solution.
d. The molarity of a solution with 1 mmol of solute in 1 L of water.
Problem 5
Select the false statement about salts.
a. Salts are ionic compounds.
b. Salts are formed when an acid and a base react with each other.
c. Salts consist of an anion and a cation component.
d. Salts may be inorganic.
e. Salts are usually acids.
f. Salts are usually hydrophilic.
Problem 6
Write the molecular formula for a substance that contains two oxygen molecules, two carbons, and four hydrogen atoms. Be sure to follow the standard conventions of writing molecular formulas.
Problem 7
Indicate the true statements and then correct the false statements so that they are true.
a. Isotopes are atoms with differing numbers of protons and the same number of neutrons.
b. A cation is a positive ion.
c. Redox reactions create ions.
d. Equal sharing of electrons leads to polar covalent bonds.
e. Ions are charged atoms.
f. CO2 is an inorganic molecule.
g. Isomers have the same molecular formula but different structures.
h. Adding a base to a solution will decrease the pH.
Problem 8
Acids donate _______________________ to an aqueous solution, which will lead to a(n) _______________________ in pH. In contrast, bases donate _______________________ to an aqueous solution and will _______________________ the pH. The pH of a solution with more OH−
than H+ will have a(n) _______________________ pH.
Problem 9
In each of the following reactions, identify the products and reactants, and state what class of reaction is shown.
Problem 10
Complete the table:
Problem 11
The notation 18O denotes a(n)
a. Isomer.
b. Isotope.
c. Dipole.
d. Ion.
e. Reaction.
Problem 12
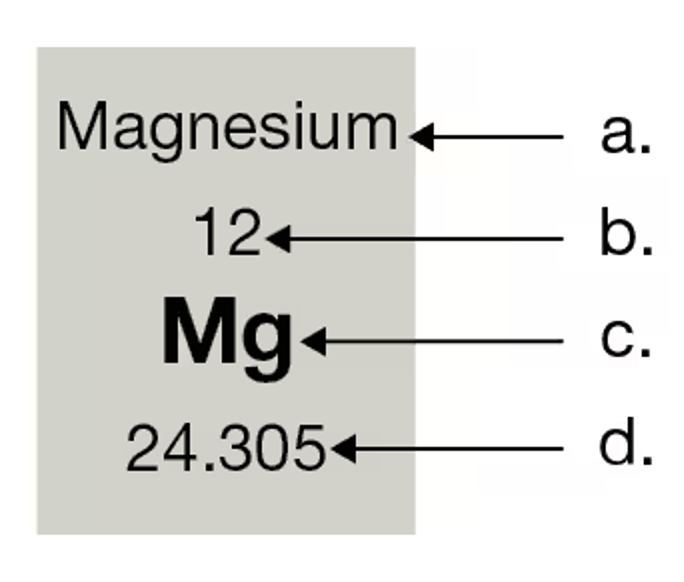
Label the features of the periodic table box:
Problem 13
How many more times acidic is a solution with a pH of 9 versus a solution with a pH of 12?
Problem 14
Which of the following is false?
a. ATP is used to fuel cellular work.
b. ATP is a specialized ribonucleotide.
c. Energy is released from ATP by breaking off phosphate groups.
d. ATP is used to fuel exergonic reactions.
Problem 15
Cholesterol is best described as
a. A lipid.
b. A sterol.
c. An alcohol.
d. A fat.
e. A wax.
Problem 16
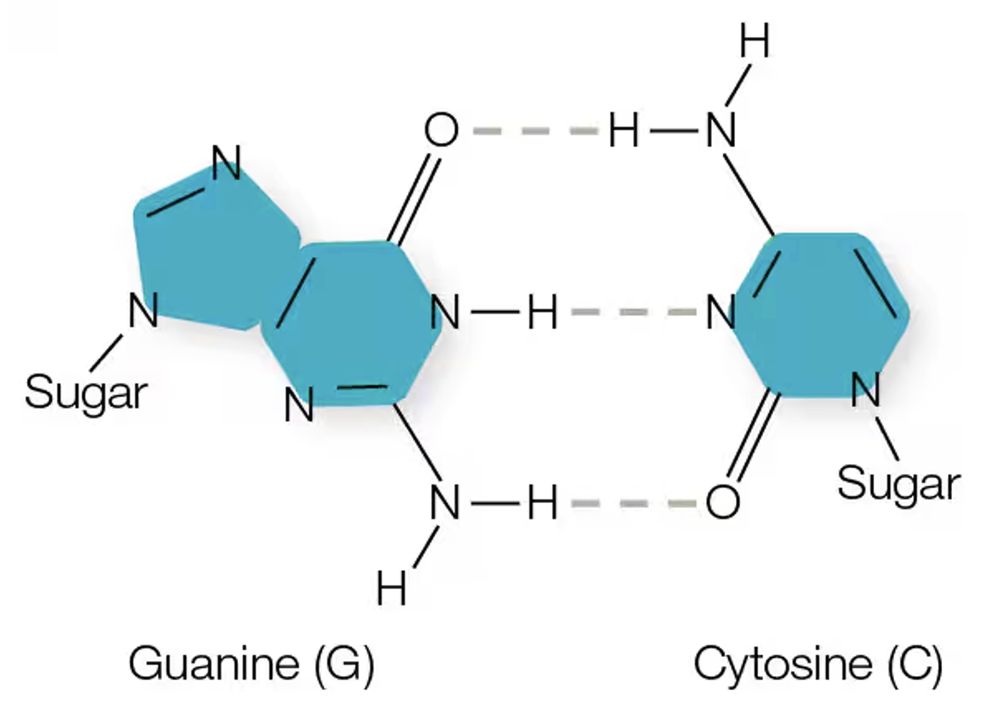
CIRCLE two covalent bonds and two hydrogen bonds in this image; label them. BOX a polar covalent bond.
Problem 17
a. b. c. d. Label the following reactions as a neutralization reaction, a hydrolysis reaction, or a dehydration synthesis reaction. <IMAGE>
Problem 18
Which of the following is/are true regarding proteins? Select all that apply.
a. Proteins are made of amino acids.
b. Proteins can have higher-order structure.
c. Proteins are made by hydrolysis reactions.
d. Peptides are large proteins.
e. A protein's secondary structure is independent of the primary structure.
f. If the protein's primary structure is altered, it will not impact the protein's tertiary structure.
Problem 19
Label the following fatty acids as saturated, monounsaturated, or polyunsaturated.