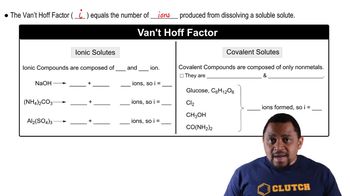
Textbook Question
A small cube of lithium 1density = 0.535 g/cm32 measuring 1.0 mm on each edge is added to 0.500 L of water. The following reaction occurs: 2 Li1s2 + 2 H2O1l2 ¡ 2 LiOH1aq2 + H21g2 What is the freezing point of the resulting solution, assuming that the reaction goes to completion?
440
views