Textbook Question
(a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days?
493
views

 Verified step by step guidance
Verified step by step guidance

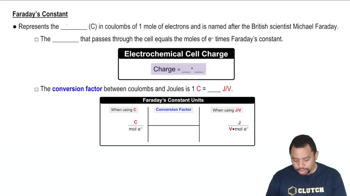

(a) A Cr3+(aq) solution is electrolyzed, using a current of 7.60 A. What mass of Cr(s) is plated out after 2.00 days?
(b) What amperage is required to plate out 0.250 mol Cr from a Cr3+ solution in a period of 8.00 h?