Textbook Question
a. Calculate the percent ionization of 0.007 M butanoic acid (𝐾𝑎=1.5×10−5).

 Verified step by step guidance
Verified step by step guidance
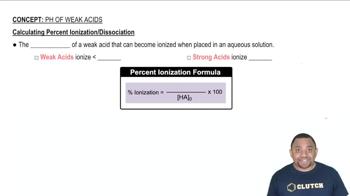


a. Calculate the percent ionization of 0.007 M butanoic acid (𝐾𝑎=1.5×10−5).
(a) Calculate the percent ionization of 0.125 M lactic acid (Ka = 1.4 × 10-4).
(b) Calculate the percent ionization of 0.125 M lactic acid in a solution containing 0.0075 M sodium lactate.