Textbook Question
(a) What is the difference between a state and a microstate of a system?
426
views

 Verified step by step guidance
Verified step by step guidance
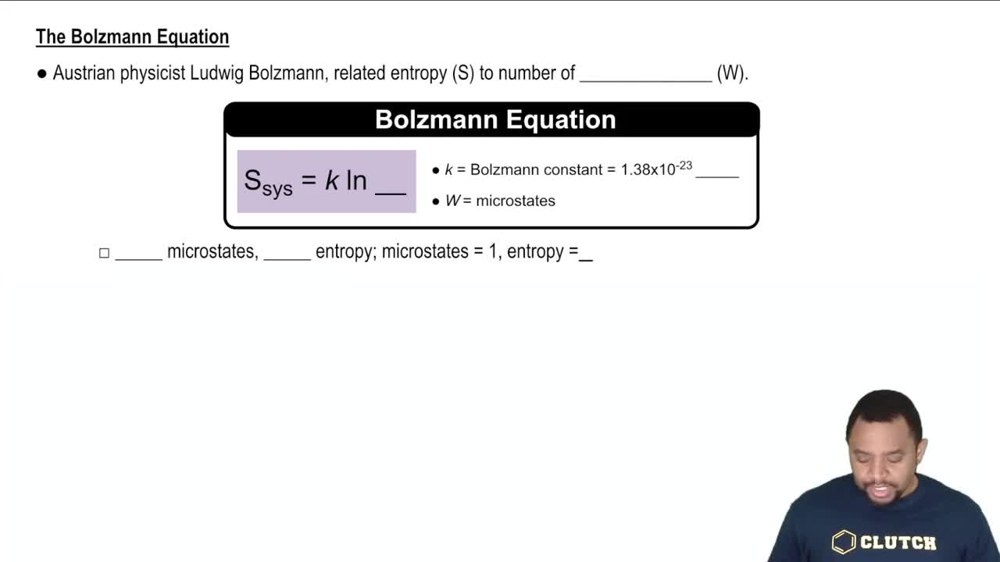
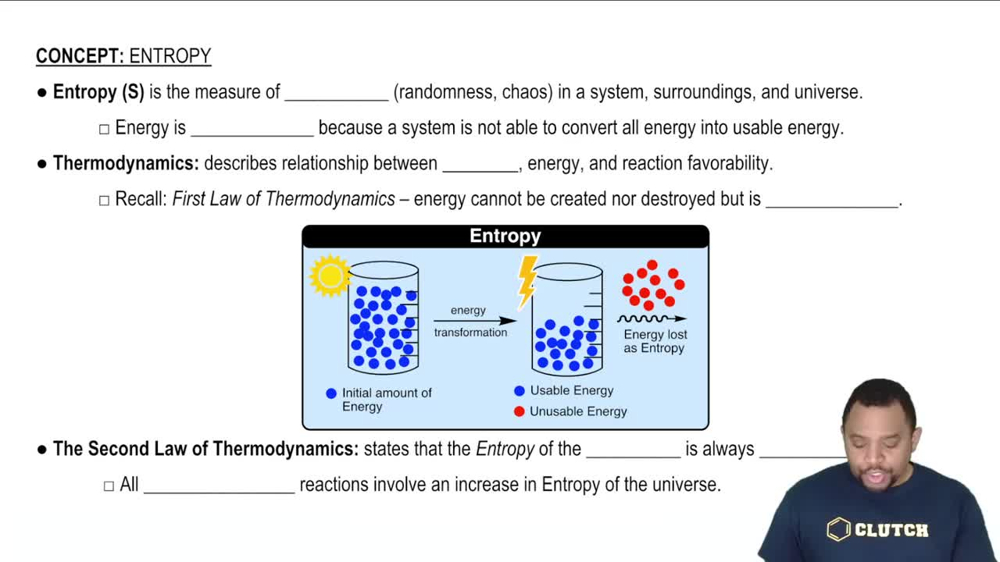
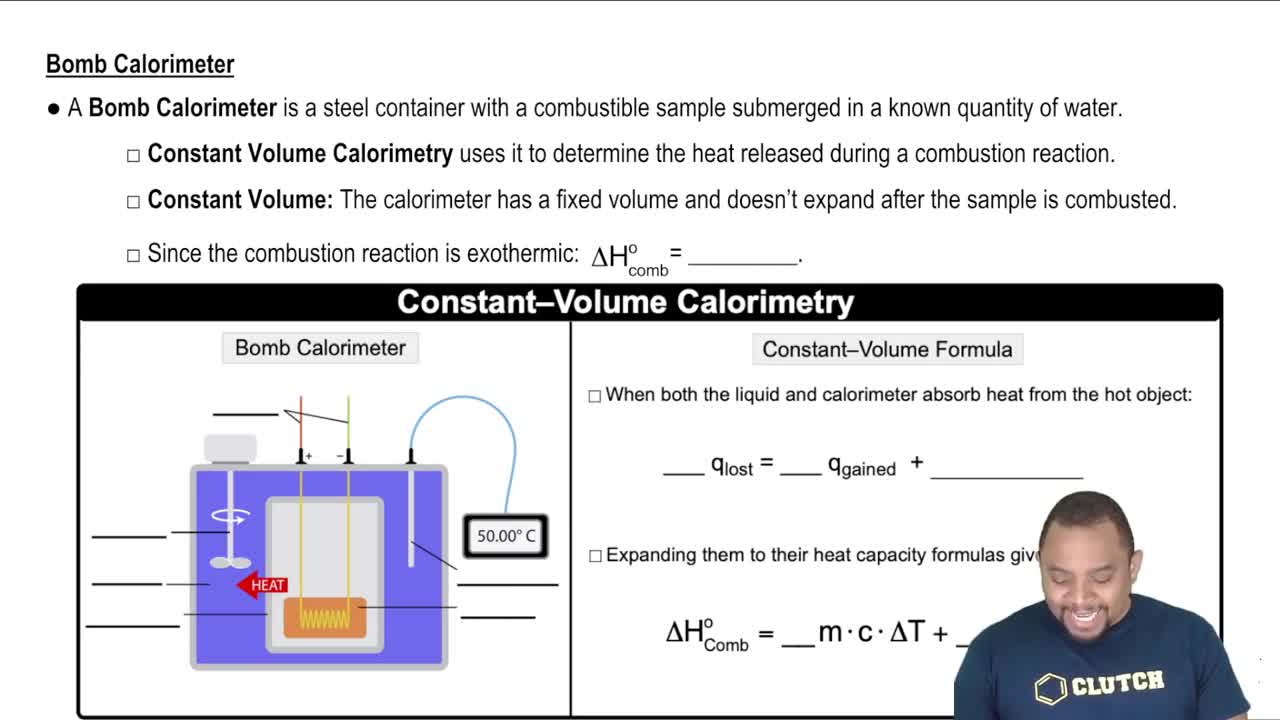
(a) What is the difference between a state and a microstate of a system?
(b) As a system goes from state A to state B, its entropy decreases. What can you say about the number of microstates corresponding to each state?
(c) In a particular spontaneous process, the number of microstates available to the system decreases. What can you conclude about the sign of ΔSsurr?
(a) In a chemical reaction, two gases combine to form a solid. What do you expect for the sign of ΔS?
(b) How does the entropy of the system change in the processes described in Exercise 19.12?