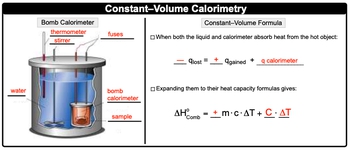
In a bomb calorimeter experiment, the heat capacity can be determined by analyzing the combustion of a known mass of a substance, in this case, ethane (C2H6). The heat of combustion for ethane is given as 1,060 kJ/mol, which is an exothermic reaction, indicating that the heat released will be negative when calculating the heat absorbed by the calorimeter.
To begin, we convert the heat of combustion from kilojoules to joules, knowing that 1 kJ equals 1,000 J. Thus, the heat of combustion becomes:
.
Next, we need to calculate the number of moles of ethane burned. Given that the mass of ethane is 12.13 grams, we first find the molar mass of ethane:
For ethane, the molar mass is calculated as follows:
- Carbon: 2 × 12.01 g/mol = 24.02 g/mol
- Hydrogen: 6 × 1.008 g/mol = 6.048 g/mol
Adding these together gives:
.
Now, we can calculate the number of moles of ethane:
.
Using the number of moles, we can calculate the heat absorbed by the calorimeter:
.
Here, the specific heat capacity of the calorimeter is approximately 52.63 J/(mol·°C), and the temperature change (ΔT) is 15.2 °C. Thus, the heat absorbed by the calorimeter can be expressed as:
.
Calculating this gives:
.
Now, applying the principle of conservation of energy, we set the heat released by the combustion equal to the heat absorbed by the calorimeter:
.
Rearranging this equation allows us to solve for the heat capacity of the calorimeter:
.
Finally, dividing both sides by the temperature change gives:
.
This value represents the heat capacity of the bomb calorimeter, indicating its ability to absorb heat during the combustion process.