Textbook Question
State whether each of these statements is true or false. e. Energy is stored in chemical bonds.
2
views

 Verified step by step guidance
Verified step by step guidance

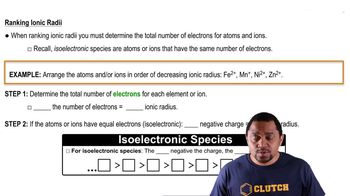
State whether each of these statements is true or false. e. Energy is stored in chemical bonds.
Consider the lattice energies of the following Group 2A compounds: BeH2, 3205 kJ/mol; MgH2, 2791 kJ/mol; CaH2, 2410 kJ/mol; SrH2, 2250 kJ/mol; BaH2, 2121 kJ/mol. (a) What is the oxidation number of H in these compounds?