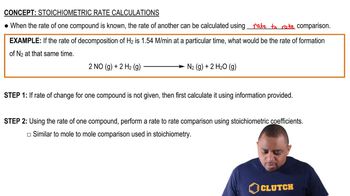
Textbook Question
A 1.00 L buffer solution is 0.250 M in HF and 0.250 M in NaF. Calculate the pH of the solution after the addition of 100.0 mL of 1.00 M HCl. (Ka = 3.5 x 10^-4) (a) 4.11(b) 3.82(c) 3.46(d) 3.09
2009
views
2
rank

 Verified step by step guidance
Verified step by step guidance