Textbook Question
Calculate the molar solubility of PbCrO4 in: (a) Pure Water(d) 1.0 x 10^-3 M K2CrO4
1134
views
1
rank

 Verified step by step guidance
Verified step by step guidance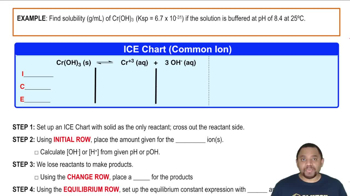


Which of the following compounds are more soluble in acidic solution than in pure water? Write a balanced net ionic equation for each dissolution reaction. (b) Fe(OH)3