Textbook Question
Examine Figure 2.3, The periodic table. (f) Classify the following three elements as metals, nonmet-als, or semimetals: Mo, Br, Si.
385
views

 Verified step by step guidance
Verified step by step guidance

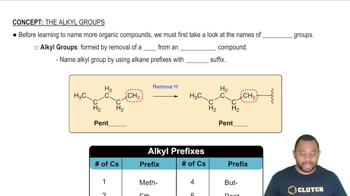
Examine Figure 2.3, The periodic table. (f) Classify the following three elements as metals, nonmet-als, or semimetals: Mo, Br, Si.
Examine Figure 2.3, The periodic table. (g) What is the symbol for the element located in period 3, group 4A?