Textbook Question
Identify the solute and the solvent in each solution composed of the following:
a. 10.0 g of NaCl and 100.0 g of H2O
1035
views

 Verified step by step guidance
Verified step by step guidance
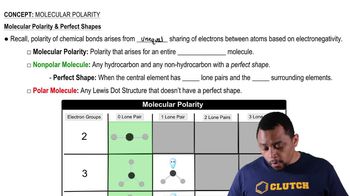

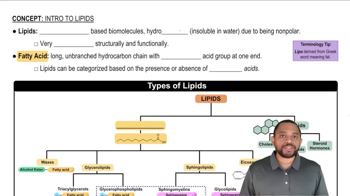
Identify the solute and the solvent in each solution composed of the following:
a. 10.0 g of NaCl and 100.0 g of H2O
Describe the formation of an aqueous KI solution, when solid KI dissolves in water.
KF is a strong electrolyte, and HF is a weak electrolyte. How is the solution of KF different from that of HF?
Write a balanced equation for the dissociation of each of the following strong electrolytes in water:
d. Fe(NO3)3
Indicate whether aqueous solutions of each of the following solutes contain only ions, only molecules, or mostly molecules and a few ions:
a. acetic acid, HC2H3O2, a weak electrolyte