Problems 49, 50, 51, and 52 show a partial motion diagram. For each: Draw a pictorial representation for your problem.

An intravenous saline drip has 9.0 g of sodium chloride per liter of water. By definition, 1 mL = 1 cm3. Express the salt concentration in kg/m3.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Concentration
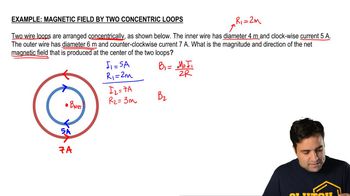
Unit Conversion

Density of Water

As an architect, you are designing a new house. A window has a height between 140 cm and 150 cm and a width between 74 cm and 70 cm. What are the smallest and largest areas that the window could be?
A 5.4 cm diameter cylinder has a length of 12.5 cm. What is the cylinder's volume in basic SI units?
The quantity called mass density is the mass per unit volume of a substance. What are the mass densities in basic SI units of the following objects? A 215 cm3 solid with a mass of 0.0179 kg.
FIGURE P1.58 shows a motion diagram of a car traveling down a street. The camera took one frame every 10 s. A distance scale is provided. Make a position-versus-time graph for the car. Because you have data only at certain instants of time, your graph should consist of dots that are not connected together.
