A flow-through electric water heater has a 20 kW electric heater inside an insulated 2.0-cm-diameter pipe so that water flowing through the pipe will have good thermal contact with the heater. Assume that all the heat energy is transferred to the water. Suppose the inlet water temperature is 12°C and the flow rate is 8.0 L/min (about that of a standard shower head). What is the outlet temperature?
Ch 19: Work, Heat, and the First Law of Thermodynamics

Knight Calc5th EditionPhysics for Scientists and EngineersISBN: 9780137344796Not the one you use?Change textbook
All textbooks Knight Calc 5th Edition
Knight Calc 5th Edition Ch 19: Work, Heat, and the First Law of Thermodynamics
Ch 19: Work, Heat, and the First Law of Thermodynamics Problem 79
Problem 79
 Knight Calc 5th Edition
Knight Calc 5th Edition Ch 19: Work, Heat, and the First Law of Thermodynamics
Ch 19: Work, Heat, and the First Law of Thermodynamics Problem 79
Problem 79Chapter 19, Problem 79
A lava flow is threatening to engulf a small town. A 400-m-wide, 35-cm-thick tongue of 1200°C lava is advancing at the rate of 1.0 m per minute. The mayor devises a plan to stop the lava in its tracks by flying in large quantities of 20°C water and dousing it. The lava has density 2500 kg/m3, specific heat 1100 J/kg K, melting temperature 800°C, and heat of fusion 4.0×105 J/kg. How many liters of water per minute, at a minimum, will be needed to save the town?
 Verified step by step guidance
Verified step by step guidance1
Calculate the volume of lava advancing per minute. The volume is given by the product of the width, thickness, and the distance the lava advances in one minute. Use the formula: \( V_{lava} = \text{width} \times \text{thickness} \times \text{distance} \).
Determine the mass of the lava advancing per minute. Use the formula: \( m_{lava} = \rho \times V_{lava} \), where \( \rho \) is the density of the lava.
Calculate the total heat that must be removed to cool the lava from its initial temperature (1200°C) to its melting point (800°C), solidify it, and then cool it further to 20°C. This involves three steps: (1) cooling the lava to its melting point using \( Q_1 = m_{lava} \cdot c \cdot \Delta T \), (2) solidifying the lava using \( Q_2 = m_{lava} \cdot L_f \), and (3) cooling the solidified lava to 20°C using \( Q_3 = m_{lava} \cdot c \cdot \Delta T \). Add these to find the total heat \( Q_{total} = Q_1 + Q_2 + Q_3 \).
Determine the amount of water required to absorb this heat. Use the formula: \( m_{water} = \frac{Q_{total}}{c_{water} \cdot \Delta T} \), where \( c_{water} \) is the specific heat of water (4186 J/kg K) and \( \Delta T \) is the temperature change of the water (from 20°C to 100°C).
Convert the mass of water per minute into liters. Since 1 kg of water is approximately equal to 1 liter, the mass of water in kilograms will directly give the volume in liters. This is the minimum amount of water needed per minute to stop the lava.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
11mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Heat Transfer
Heat transfer is the process by which thermal energy moves from one object or substance to another. In this scenario, the heat from the lava must be absorbed by the water to cool it down. Understanding the mechanisms of conduction, convection, and radiation is essential for calculating how much water is needed to effectively stop the advancing lava.
Recommended video:
Guided course

Overview of Heat Transfer
Specific Heat Capacity
Specific heat capacity is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius. In this case, the specific heat of the lava and water will determine how much energy must be absorbed by the water to cool the lava from 1200°C to below its melting temperature of 800°C. This concept is crucial for calculating the necessary water volume.
Recommended video:
Guided course

Specific Heat & Temperature Changes
Density and Volume Calculations
Density is defined as mass per unit volume and is critical for converting between mass and volume in calculations. The density of the lava and water will help determine how much mass of water is needed to absorb the heat from the lava. By understanding the relationship between mass, volume, and density, one can accurately compute the liters of water required to mitigate the lava flow.
Recommended video:
Guided course
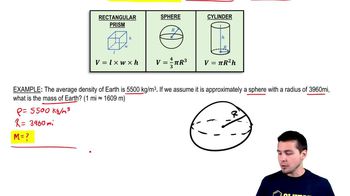
Problems with Mass, Volume, & Density
Related Practice
Textbook Question
704
views
Textbook Question
In Problems 75, 76, and 77 you are given the equation used to solve a problem. For each of these, you are to write a realistic problem for which this is the correct equation.
86
views
Textbook Question
10 g of aluminum at 200°C and 20 g of copper are dropped into 50 cm3 of ethyl alcohol at 15°C. The temperature quickly comes to 25°C. What was the initial temperature of the copper?
996
views
Textbook Question
FIGURE CP19.80 shows a thermodynamic process followed by 0.015 mol of hydrogen. How much heat energy is transferred to the gas?
828
views