Textbook Question
The power output of a car engine running at 2400 rpm is 500 kW. How much (a) work is done and (b) heat is exhausted per cycle if the engine's thermal efficiency is 20%? Give your answers in kJ.
1029
views

 Verified step by step guidance
Verified step by step guidance


The power output of a car engine running at 2400 rpm is 500 kW. How much (a) work is done and (b) heat is exhausted per cycle if the engine's thermal efficiency is 20%? Give your answers in kJ.
A gas following the pV trajectory of FIGURE EX21.11 does 60 J of work per cycle. What is Vmax?
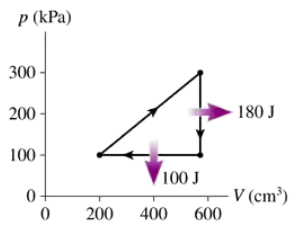
What are (a) Wout and QH and (b) the thermal efficiency for the heat engine shown in FIGURE EX21.14?
A 15 kW electric generator burns 1.2 gal of diesel fuel per hour. The energy density of diesel fuel is 140 MJ/gal. What is the generator's thermal efficiency?
An air conditioner removes 5.0 x 10⁵ J/min of heat from a house and exhausts 8.0 x 10⁵ J/min to the hot outdoors. What is the air conditioner's coefficient of performance?