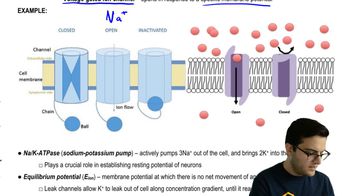
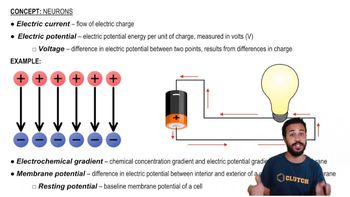
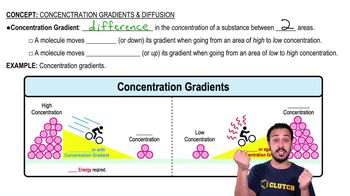
Consider the following statements regarding limiting nutrients. Select True or False for each statement.
T/F Nitrogen (N), phosphorus (P), and potassium (K) are common examples.
T/F Their presence limits the availability of micronutrients.
T/F Their availability tends to limit plant growth.
T/F Certain macronutrients and micronutrients can be considered limiting nutrients.