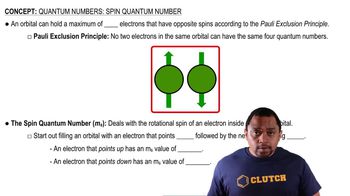
The human retina has three types of receptor cones, each sensitive to a different range of wavelengths of visible light, as shown in this figure (the colors are merely to differentiate the three curves from one another; they do not indicate the actual colors represented by each curve):
(c) Explain why the sky appears blue even though all wavelengths of solar light are scattered by the atmosphere.