11. Bonding & Molecular Structure
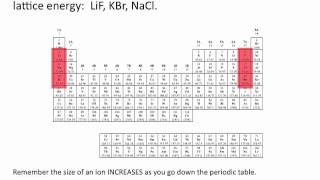
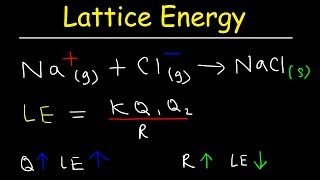
Lattice Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 22b
Textbook Question
Textbook QuestionNaCl and KF have the same crystal structure. The only difference between the two is the distance that separates cations and anions. (a) The lattice energies of NaCl and KF are given in Table 8.1. Based on the lattice energies, would you expect the Na¬Cl or the K¬F distance to be longer?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1193
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos