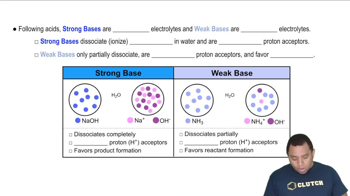
Textbook Question
The following pictures represent solutions of Ag2CrO4, which also may contain ions other than Ag+ and CrO42- that are not shown. Solution 1 is in equilibrium with solid Ag2CrO4. Will a precipitate of solid Ag2CrO4 form in solutions 2-4? Explain.
(1) (2) (3) (4)
584
views
1
rank