15. Chemical Kinetics
Average Rate of Reaction

Get help from an AI Tutor
Ask a question to get started.
Problem 20
Textbook Question
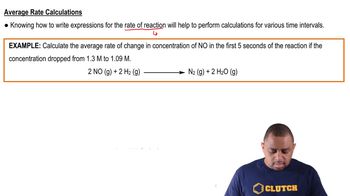
Textbook QuestionA flask is charged with 0.100 mol of A and allowed to react to form B according to the hypothetical gas-phase reaction A1g2¡B1g2. The following data are collected: Time (s) 0 40 80 120 160 Moles of A 0.100 0.067 0.045 0.030 0.020 (c) Which of the following would be needed to calculate the rate in units of concentration per time: (i) the pressure of the gas at each time, (ii) the volume of the reaction flask, (iii) the temperature, or (iv) the molecular weight of A?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
830
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos