12. Molecular Shapes & Valence Bond Theory
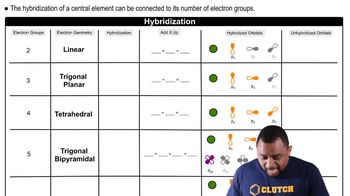
Hybridization

Get help from an AI Tutor
Ask a question to get started.
Problem 31
Textbook Question
Textbook QuestionThe VSEPR model is a simple predictive tool that is usually, but not always, correct. Take urea, for instance, a waste product excreted in animal urine:

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
421
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos