Textbook Question
Calculate the pH of each of the following strong acid solutions: (b) 1.52 g of HNO3 in 575 mL of solution
867
views

 Verified step by step guidance
Verified step by step guidance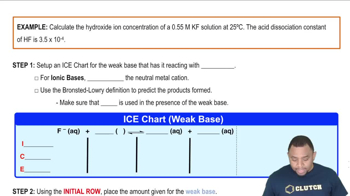


Calculate the pH of each of the following strong acid solutions: (b) 1.52 g of HNO3 in 575 mL of solution
Calculate the pH of each of the following strong acid solutions: (b) 0.225 g of HClO3 in 2.00 L of solution
Calculate [OH-] and pH for each of the following strong base solutions: (c) 10.0 mL of 0.0105 M Ca(OH)2 diluted to 500.0 mL