Arrange the following 0.10 M solutions in order of increasing acidity: (i) NH4NO3, (ii) NaNO3, (iii) CH3COONH4, (iv) NaF, (v) CH3COONa.

Many moderately large organic molecules containing basic nitrogen atoms are not very soluble in water as neutral molecules, but they are frequently much more soluble as their acid salts. Assuming that the pH in the stomach is 2.5, indicate whether each of the following compounds would be present in the stomach as the neutral base or in the protonated form: nicotine, Kb = 7 * 10-7; caffeine, Kb = 4 * 10-14; strychnine, Kb = 1 * 10-6; quinine, Kb = 1.1 * 10-6.
 Verified step by step guidance
Verified step by step guidanceKey Concepts
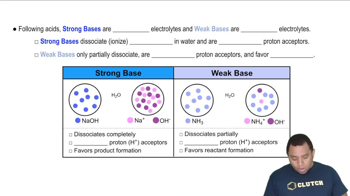
Acid-Base Chemistry

pKa and Kb Relationship

Solubility of Salts vs. Neutral Bases
The following observations are made about a diprotic acid H2A: (i) A 0.10 M solution of H2A has pH = 3.30. (ii) A 0.10 M solution of the salt NaHA is acidic. Which of the following could be the value of pKa2 for H2A: (i) 3.22, (ii) 5.30, (iii) 7.47, or (iv) 9.82?
The amino acid glycine (H2N–CH2–COOH) can participate in the following equilibria in water:
H2N–CH2–COOH + H2O ⇌ H2N–CH2–COO– + H3O+ Ka = 4.3 × 10-3
H2N–CH2–COOH + H2O⇌ +H3N–CH2–COOH + OH- Kb = 6.0 × 10-5
(a) Use the values of Ka and Kb to estimate the equilibrium constant for the intramolecular proton transfer to form a zwitterion: H2N–CH2–COOH ⇌ +H3N–CH2–COO–
The amino acid glycine (H2N–CH2–COOH) can participate in the following equilibria in water:
H2N–CH2–COOH + H2O ⇌ H2N–CH2–COO– + H3O+ Ka = 4.3 × 10-3
H2N–CH2–COOH + H2O⇌ +H3N–CH2–COOH + OH- Kb = 6.0 × 10-5
(b) What is the pH of a 0.050 M aqueous solution of glycine?
